
Company
 About Us About Us
 Management Management
 Advisors Advisors
 Contact Us Contact Us
Technology
 APOE and Human APOE and Human
 Disease Disease
 APOE and APOE and
 Inflammation Inflammation
 Cognosci APOE Cognosci APOE
 Compounds Compounds
 Publications Publications
Pipeline
 Multiple Sclerosis Multiple Sclerosis
 Traumatic Brain Traumatic Brain
 Injury Injury
 Subarachnoid Subarachnoid
 Hemorrhage Hemorrhage
 Rheumatoid Arthritis Rheumatoid Arthritis
 Ischemic Stroke Ischemic Stroke
 Sepsis Sepsis
 Alzheimer’s Disease Alzheimer’s Disease
 Parkinson’s Disease Parkinson’s Disease
Partnering
 Multiple Sclerosis Multiple Sclerosis
 Alzheimer’s Disease Alzheimer’s Disease
 Subarachnoid Subarachnoid
 Hemorrhage Hemorrhage
News
 Latest News Latest News
|

|
Pipeline
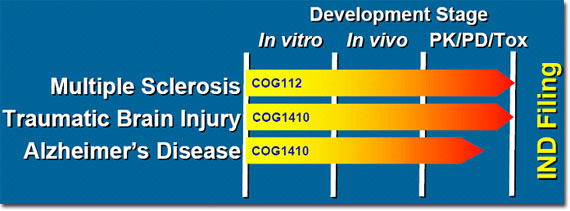
Cognosci Inc. is focused on development of drugs to treat diseases with a pharmacogenomic linkage to the apolipoprotein E (apoE) protein including Multiple Sclerosis, Alzheimer's Disease, and Traumatic Brain Injury. For most of these diseases, people with the 4 allele of apoE typically suffer worse outcomes. Scientists from Cognosci have created and optimized novel apoE-based compounds with potent anti-inflammatory and neuroprotective activities using more than $7.5 million in Phase I and Phase II Small Business Innovation Resource (SBIR) grants from the National Institutes of Health. These funds have been used to identify lead candidates for each of the diseases described below with projects ranging from lead identification to full preclinical development programs.
Current Leads
Multiple Sclerosis
Multiple Sclerosis (MS) is widely recognized as an autoimmune disorder that poses a significant medical challenge. Autoimmune disorders occur when the immune system, which normally functions to fight viral and bacterial challenges, undergoes aberrant activation to recognize human proteins as antigens. This aberrant activation results in attack of the immune system on structures in the body rather than invading infective agents. In the case of MS, one of the antigens is the myelin protein that insulates nerve fibers in the brain and spinal cord, which leads to destruction of the myelin sheath. Myelin is critical for nerve signaling and it's destruction results loss of nerve signal conductance and eventually, to nerve cell death. The neurodegeneration caused by the immune attack results in a progressive loss of neuromuscular control, increasing disability, and eventually death.
 Cognosci has developed the first neuro-restorative compounds for treatment of Multiple Sclerosis that display a significant reduction of disability in animal models of MS. These compounds are small synthetic peptides of ten to seventeen amino acids that are based on the multifunctional apoE protein, and have potent in vitro and in vivo anti-inflammatory activity. In addition to inhibiting inflammation, Cognosci's "COG" compounds also directly protect nerve cells by blocking activity of endogenous neurotoxins released by injured brain cells. In the case of Multiple Sclerosis, the dual actions (immune suppression and direct neuroprotection) of our lead compounds are further bolstered by neuro-reparative activity where the compounds promote repair of damaged nerve cells to restore normal function.
Cognosci has developed the first neuro-restorative compounds for treatment of Multiple Sclerosis that display a significant reduction of disability in animal models of MS. These compounds are small synthetic peptides of ten to seventeen amino acids that are based on the multifunctional apoE protein, and have potent in vitro and in vivo anti-inflammatory activity. In addition to inhibiting inflammation, Cognosci's "COG" compounds also directly protect nerve cells by blocking activity of endogenous neurotoxins released by injured brain cells. In the case of Multiple Sclerosis, the dual actions (immune suppression and direct neuroprotection) of our lead compounds are further bolstered by neuro-reparative activity where the compounds promote repair of damaged nerve cells to restore normal function.
These lead compounds are the first neuro-restorative compounds with this combination of anti-inflammatory, neuroprotective, and neuro-reparative activity. These compounds treat both the relapsing-remitting and progressive forms of MS, unlike currently approved drugs that treat only the relapsing-remitting form of MS. Cognosci anticipates entry of a lead candidate into full safety assessment by Q3 2007 and will be ready for human clinical trials in 2008.
Cognosci has multiple lead compounds that show efficacy in animal models of both relapsing-remitting and progressive MS. The figures below show the clinical course of disability over time in progressive and relapsing-remitting EAE models of MS. Control animals received only saline injections and experienced higher clinical scores that are indicative of a higher degree of clinical disability. In the progressive model, sick animals treated with Cognosci compounds starting when clinical scores of disability reached a value of 2, which is indicative of hind limb paralysis and gait disturbance, showed a significant improvement in clinical scores with virtually complete recovery for some of the treatment groups.
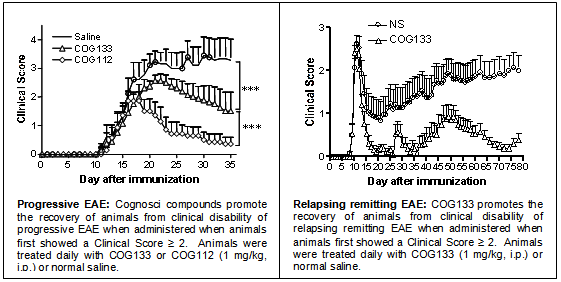
COG133 treatment also demonstrates a significant clinical improvement in the PLP-induced EAE model of relapsing remitting MS, including complete remission of clinical signs in 25% of the treated animals. Additionally, COG133 extended the average time between relapses from 12 days in control animals to 28 days in COG133 treated animals. This 60% reduction in the relapse rate was also accompanied by a 70% reduction in total disability in treated mice. Histopathological analysis of the spinal cords from the progressive EAE animals revealed no significant demyelinated lesions or infiltration in compound treated animals whereas large demyelinated lesions with infiltrating T-cells were prominent in the control animals (see histology slides below).
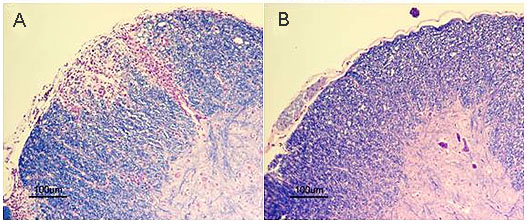
Histopathological Analysis: COG133 prevented spinal cord demyelination in MOG-induced EAE mice. The animals were sacrificed 30 days after MOG immunization and the whole spinal cord was dissected out and 5- m -thick sections were made from cervical segments of COG133-treated animals (B) and Normal Saline treated ones (A). These sections were stained with Luxol fast blue (for myelin, stained in blue) and then counterstained with eosin (showing peripheral infiltrates, in purple).
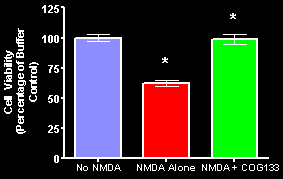
The ability of the Cognosci compounds to reduce disability is derived from suppression of inflammation, protection of damaged neurons from endogenous neurotoxins release by degeneration of myelin producing cells, and promoting remyelination of damaged neurons. The anti-inflammatory effects are driven by inhibition of microglial activation, reducing secretion of proinflammatory cytokines, and suppression of nitric oxide production in both peripheral tissues and in the CNS (see left panel below; Laskowitz et al. 2001 for in vitro studies and Lynch et al. 2003 for in vivo studies). These compounds also protect neurons from NMDA excitotoxicity (see graph below; Aono et al. 2003).
|
Cultured primary cerebellar granular cells obtained from day 8 post-natal rat pups were pre-treated with buffer or COG133 (2.5 M) for one hour before exposure to 100 M NMDA. Cell viability was determined with an MTT assay at 24 hr. Bars represent the mean ± S.D. of 6 replicates of 6 and COG133 p < 0.05 compared to cells treated with NMDA.
|

|
|
|

|
Latest News
Cognosci Receives Funding for Advanced Testing of Lead Drug Candidate for the Treatment of Multiple Sclerosis
more...
For more information, contact:
Cognosci Inc.
79 T. W. Alexander Drive
4401 Research Commons
Research Triangle Park
NC 27709
(919) 765-0028
|



